Precision Medicine For NAFLD
21st October 2021, 2pm-4:30pm
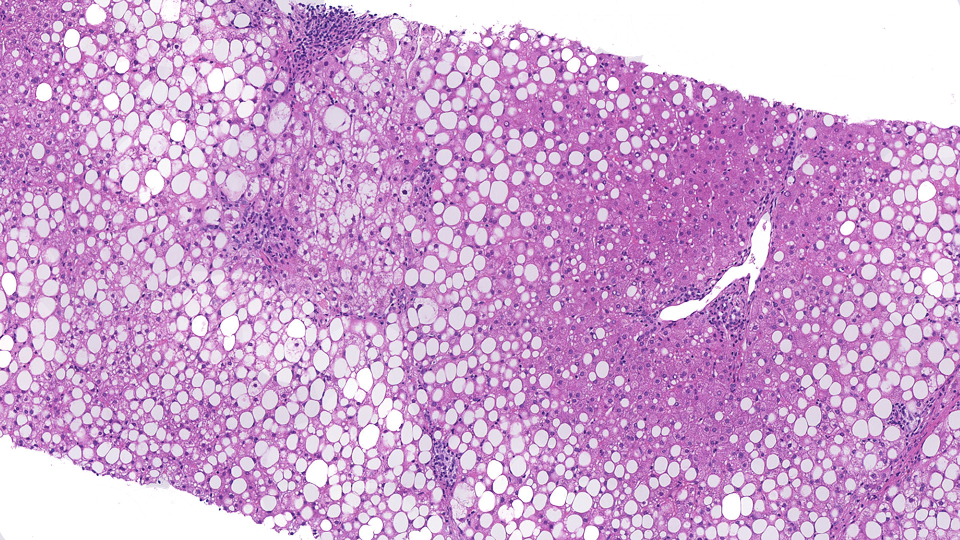
Join us to learn more about the creation of the world’s first data commons for Nonalcoholic Fattly Liver Disease (NAFLD) – ‘SteatoSITE’. SteatoSite is a